Pharmacopée Européenne – 12e Édition exclusivement en ligne

Les nouvelles licences valables 365 jours seront disponibles à la commande à partir de mai 2025
La principale source normative en matière de contrôle qualité
La Pharmacopée Européenne (Ph. Eur.) constitue la principale source de normes qualité officielles applicables aux médicaments et à leurs ingrédients en Europe. Ces normes fournissent une base juridique et scientifique au contrôle qualité d’un produit tout au long de son cycle de vie et soutiennent ainsi l’industrie pharmaceutique et les systèmes de santé.
Comme stipulé dans la Convention du Conseil de l’Europe relative à l’élaboration d’une Pharmacopée européenne et dans les législations pharmaceutiques nationales et de l’Union européenne, ces normes sont juridiquement contraignantes. Tous les fabricants pharmaceutiques sont donc tenus de les appliquer pour pouvoir commercialiser leurs produits dans les États signataires de la Convention.
Commandez une licence valable 365 jours pour suivre l’évolution de la Ph. Eur.
Protection de la santé publique
L’EDQM compile et publie la Ph. Eur. conformément à ses obligations décrites dans la Convention et à sa mission de protection de la santé publique. Grâce à leur expertise, scientifiques, universitaires, fonctionnaires des autorités réglementaires et autres professionnel·les du domaine du médicament contribuent à établir les normes de la Ph. Eur., qui permettent à des millions de personnes d’accéder à des médicaments de qualité. La Commission européenne de Pharmacopée est l’organe de décision chargé d’adopter les textes de la Ph. Eur. et de prendre des décisions d’ordre technique par consensus.
En savoir plus sur la Commission européenne de Pharmacopée.
Rejoignez la communauté de la Ph. Eur. et partagez votre expertise : ensemble, nous pouvons donner vie à notre vision d’une meilleure santé pour tout le monde.
Quoi de neuf dans cette 12e Édition ?
À compter de la 12e Édition, la Ph. Eur. sera disponible exclusivement en ligne.
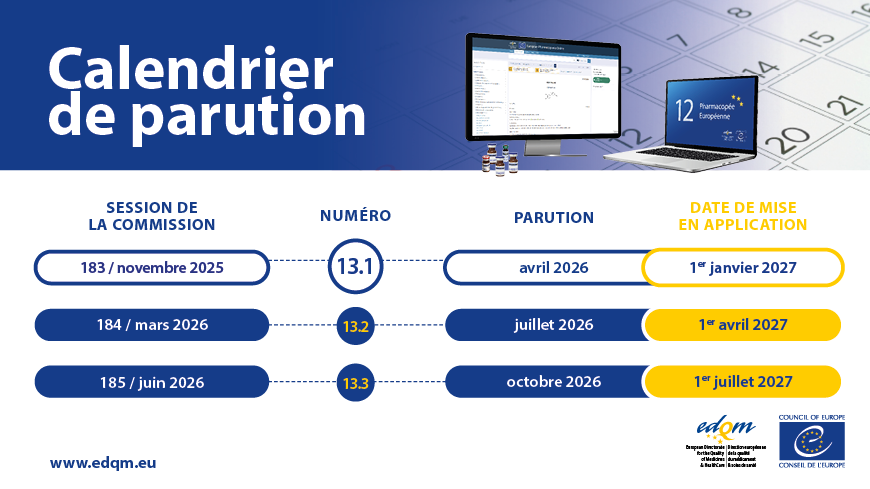
Le cycle précédent de trois ans, qui comprenait une édition et huit suppléments, sera remplacé par une édition annuelle composée de trois numéros. La 12e Édition est ainsi composée des Numéros 12.1, 12.2 et 12.3. Chacun des numéros contiendra les textes (nouveaux et révisés) adoptés lors de l’une des trois sessions annuelles de la Commission européenne de Pharmacopée (EPC).
Le modèle de licence a également évolué. Désormais plus pratique, il s’agit d’une licence valable 365 jours.
Une nouvelle plateforme en ligne pour la Ph. Eur.
La nouvelle Ph. Eur. en ligne offre :
- un accès aux versions cumulées de la Ph. Eur. (en anglais et en français) ;
- la compatibilité avec les dernières versions des navigateurs Edge, Chrome, Firefox et Safari ;
- pour la première fois, au même endroit, l’accès à l’ensemble des textes actuellement en vigueur et des textes publiés depuis 11.0 ;
- un menu pour naviguer entre les différentes versions d’un texte directement depuis celui-ci ;
- un code couleur pour indiquer le statut des textes et vous aider à mieux vous y retrouver :
- « en vigueur » (vert) : textes juridiquement contraignants et mis en application ;
- « n’est plus en vigueur » (rouge) : textes supprimés ou remplacés par des versions plus récentes ;
- « bientôt en vigueur » (orange) : textes qui ne sont pas encore mis en application ;
- un accès continu aux archives en ligne de la Ph. Eur. et aux informations complémentaires répertoriées pour chaque texte dans la base de données Knowledge ;
- de nouvelles fonctions améliorées de recherche et de filtrage par titre, par numéro de texte, par statut et par type de texte ;
- des recherches prédéfinies pour consulter, en un clic, toutes les nouveautés d’un numéro, et une liste intitulée « Nouveau contenu » contenant les liens vers l’ensemble des chapitres généraux, des monographies ou des réactifs publiés dans un numéro ;
- des fonctions de gestion de licence actualisées, avec notamment l’affichage du nombre d’accès utilisateur disponibles et de la durée d’utilisation restante de la licence.
Inscrivez-vous à notre webinaire pour découvrir toutes les fonctionnalités conviviales de la nouvelle Ph. Eur. en ligne et obtenir des astuces afin de trouver rapidement et efficacement les informations dont vous avez besoin.
Prix et offres spéciales
- 650 € par licence individuelle (accès de 365 jours) ; remise accordée en cas d’achat simultané de plusieurs licences individuelles (voir le barème de remise) ;
- 70 000 € par licence d’accès illimité ;
- 300 € par licence université (offre spéciale applicable uniquement aux universités).
Pour plus d’informations, consultez la liste des prix de la 12e Édition et reportez-vous à la section Informations Ventes ci-contre.