From feasibility study to pilot phase
From the outset, the EDQM considered that a live, real-time video connection between the inspectors and the facilities to be inspected would be the best possible means of bridging the two existing approaches and enabling GMP assessments to continue despite travel restrictions. Setting up such a real-time video connection involves serious technical obstacles, which were considered as challenging. However, in view of the potential benefits and the travel restrictions, the EDQM decided to launch a pilot phase. In September 2020, the EDQM reached out to manufacturing sites that had already been inspected by the EDQM and had a good GMP-compliance record to find volunteers.
Real-time remote inspections require more thorough preparation than on-site inspections. For example, it was necessary to hold preparatory teleconferences with the companies volunteering to participate, followed by a connectivity test (or “dry run”) to ensure that all areas to be inspected were equipped with the necessary networks. Depending on the circumstances, and taking into account the technical solutions available at each specific location, the participating companies set up Wi-Fi networks, cellular networks or hybrid networks comprising elements of these technologies. It was found that a data-transmission rate over 100 kilobytes/second was sufficient to achieve a steady video transmission. This was usually the case when using broadband LAN/WLAN in meeting rooms, but sometimes represented a challenge when using wireless or cellular networks in manufacturing areas.
For communication, sharing of live video footage and review of documents, the EDQM used secure web conferencing applications, which present the least security issues. A primary and a secondary web conferencing application were selected for use during inspections, both as part of a redundancy strategy and to allow inspectors to work in parallel. Both web conferencing tools could be installed on mobile devices with web cameras, which could then be used during the inspection.
The real-time, secure sharing of confidential documents also required some thought. The EDQM used a secure document sharing tool offering a secure storage space for documents and other files, physically located on its premises. The participating companies were given access to this tool to upload documents requested before and during the inspection.
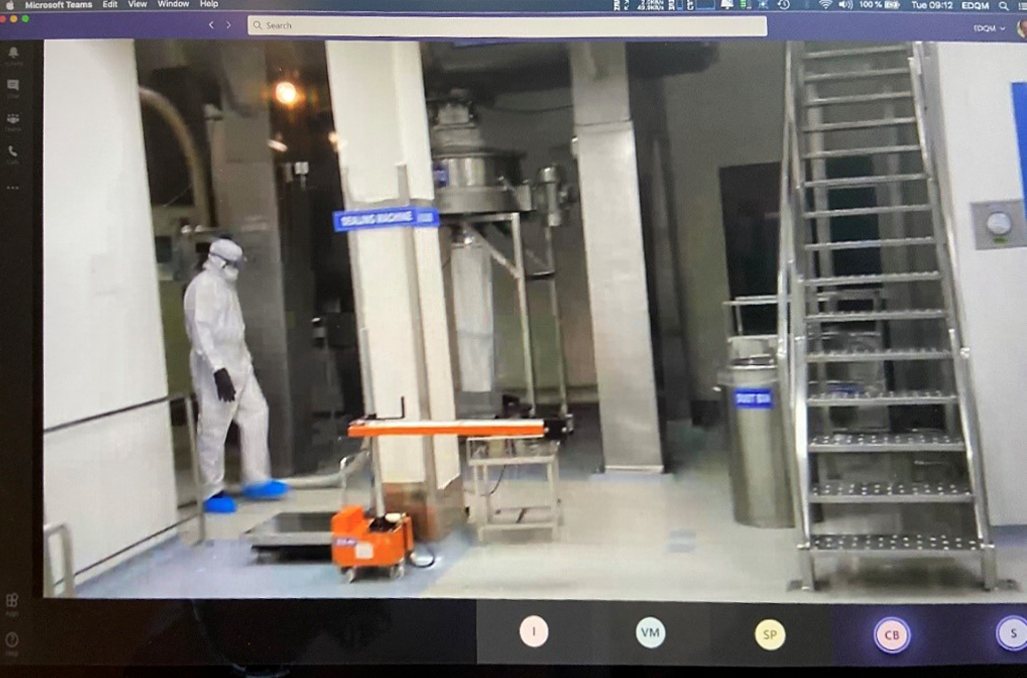
Another important technical issue was to determine what kind of devices could be used to capture live footage from potentially hazardous manufacturing areas. Upon investigation, it was found that “intrinsically safe” mobile phones (incapable of causing ignition of hazardous atmospheric mixtures) were already used in explosive atmospheres such as in refineries. During the inspections carried out in the pilot phase, companies used various devices suited to their needs, such as cell phones, tablet computers and head-mounted devices, all equipped with operating systems allowing the installation of web conferencing applications.
Most CEP-holding manufacturers are located in Asia. To minimise the time-difference impact for remote inspections, Indian companies were selected for the pilot phase. Both parties needed to make an effort during remote meetings taking place across several time zones in order to make the best use of the agreed inspection slot. The companies inspected were very open-minded and an inspection rhythm of six to seven hours per day over several days was established. Participating inspectors were nevertheless unanimously of the opinion that one or two days of jetlag is easier to deal with than getting up very early for an entire week. Furthermore, the inspection team worked in parallel sessions whenever possible to achieve the inspection’s objectives and save time.