European Pharmacopoeia – New online-only 12th Edition

New 365-day access licences available to order from May 2025
The primary source for quality control standards
The European Pharmacopoeia (Ph. Eur.) is the primary source of official quality standards for medicines and their ingredients in Europe. Ph. Eur. standards provide a legal and scientific basis for the quality control of a product throughout its life cycle, supporting the pharmaceutical industry and healthcare systems.
As laid down in the Council of Europe Convention on the Elaboration of a European Pharmacopoeia and in European Union and national pharmaceutical legislation, these standards are legally binding. All producers of pharmaceuticals must therefore apply them in order to market their products in the signatory states of the Convention.
Order a 365-day access licence and stay connected with the Ph. Eur.
Public health protection
The Ph. Eur. is compiled and published by the EDQM as part of its obligations under the Convention and its public health protection mission. Scientists, academics, regulatory officials and others working with medicines contribute their expertise to help establish the Ph. Eur. standards that make it possible for millions of people to have access to quality medicines. The European Pharmacopoeia Commission is the decision-making body responsible for adopting Ph. Eur. texts and making technical decisions by consensus.
Learn more about the European Pharmacopoeia Commission.
Join the Ph. Eur. family to share your expertise: together, we can achieve our vision of better health for all.
New for the 12th Edition
As of the 12th Edition, the Ph. Eur. will be available in an online-only format.
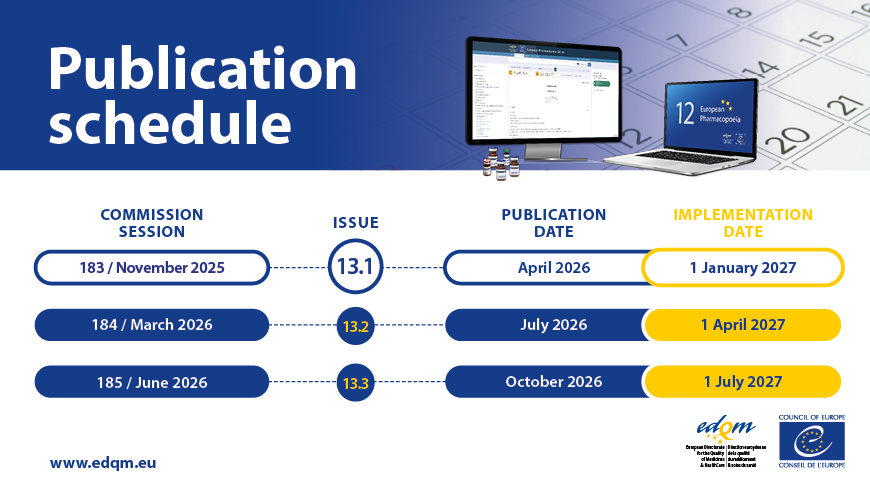
The previous three-year cycle, which included one edition and eight supplements, is being replaced by an annual edition consisting of three issues. The 12th Edition is therefore composed of Issues 12.1, 12.2, and 12.3. Each issue will contain new and revised texts adopted at one of the three European Pharmacopoeia Commission (EPC) sessions held annually.
The licence model has also been switched to a user-friendly 365-day licence.
A new online platform for the Ph. Eur.
The new Ph. Eur. platform offers:
- access to fully cumulative versions (English and French) of the Ph. Eur.;
- compatibility with recent Edge, Chrome, Firefox and Safari browsers;
- for the first time, access to all texts currently in force and texts published since 11.0, in one convenient space;
- an in-text menu to navigate between different versions of a text
- a new text status traffic light system to help you find the texts you need quickly and efficiently:
- “in force” (green) for legally binding, implemented texts;
- “no longer in force” (red) for texts that have been suppressed or superseded by newer versions;
- “not yet in force” (orange) for texts that have not yet reached their implementation date;
- direct links to versions of texts undergoing public consultation in Pharmeuropa
- continued access to the Ph. Eur. online archives and complementary information on texts in the Knowledge database
- new and improved features for searching and filtering by title, text number, text status, and text type;
- predefined searches to see at a glance what's new in an issue and a New Content list with links to all general chapters, monographs and reagents published in an issue;
- updated licence management features such as counters displaying the number of remaining user accesses available and time left on the licence.
Sign up to our webinar to discover all the user-friendly functionalities of the new Ph. Eur. Online, as well as tips to help you find the information you need quickly and efficiently .
Prices and offers
- 650€ for an individual licence (365-day access); discount granted for two or more individual licences bought simultaneously (see discount scale);
- 70 000€ for an unlimited user licence;
- 300€ for a university licence (special offer for universities only).
For more information, consult the 12th Edition price list and see “Sales information” opposite.