European Pharmacopoeia (Ph. Eur.)
Find your way - Topics
The European Pharmacopoeia
Groups of experts and working parties
European Pharmacopoeia 11th Edition
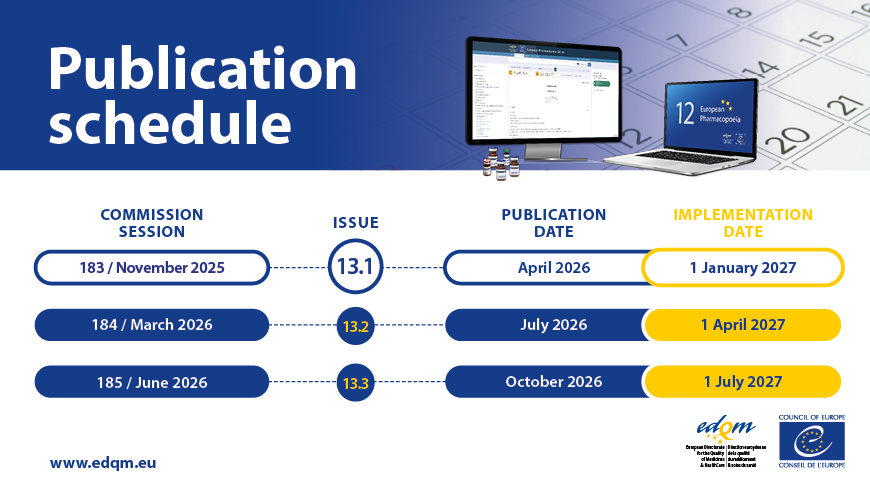
European Pharmacopoeia 12th Edition
Focus
Alternatives to animal testing (3Rs)
How to participate in the work of the Ph. Eur.
The Ph. Eur. work programme
Where to find: the Knowledge database
Pharmacopoeial Harmonisation
Harmonisation status for Excipient monographs (PDG)
Harmonisation status for General Texts (PDG)
Ph. Eur. reference standards
Biological standardisation programme (BSP)
Find information on
EDQM Newsletter
Click here to subscribe.