Quality Management
In order to sustain our vision and mission and to guarantee the best products and services to our customers and stakeholders, the EDQM implements a Quality Management System applying the seven quality management principles of ISO 9001:
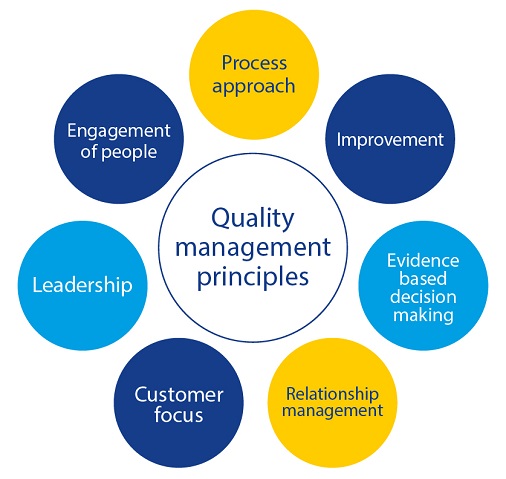
In line with the requirements of ISO 9001:2015, the EDQM deploys risk-based thinking to the planning and implementation of its Quality Management System processes. Addressing risks and opportunities enhances its effectiveness, improves results and prevents negative effects.
All these principles guide how the EDQM works across all its activities, enabling it to meet the needs of stakeholders and stay agile in a fast-moving and constantly changing environment. They support the delivery of strategic objectives and the continued growth of the organisation.
The EDQM laboratory is accredited for ISO/IEC 17025:2017 – “General requirements for the competence of testing and calibration laboratories”, demonstrating that the laboratory operates a quality system, is technically competent and is able to generate technically valid results.
This standard covers all aspects of laboratory management, including sample preparation, analytical testing competence, documentation control, facilities and environmental conditions, equipment, traceability and reporting.
The EDQM laboratory has been officially accredited for the 2017 version of ISO/CEI 17025 since February 2020, having previously been accredited for the 2005 version since 2013.
The EDQM laboratory ISO/CEI 17025 accreditation certificate covers 16 techniques as described in the annex of the certificate.



