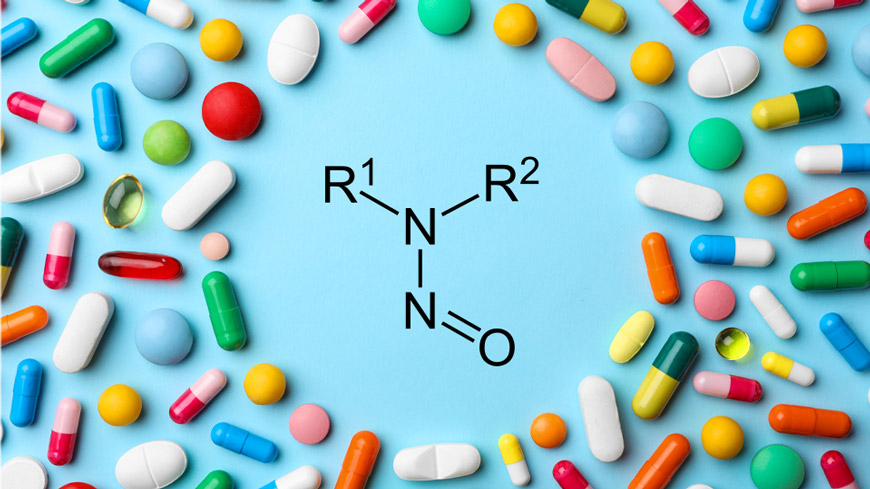
At its 177th session in November 2023, the European Pharmacopoeia Commission (EPC) approved the strategy for N-nitrosamine impurities in individual monographs.
Regarding active substances
The EPC agreed to delete the Production section covering N-nitrosamine impurities from existing individual monographs on active substances and to avoid including such statements in new monographs in the future, since the general requirement for these impurities given in revised general monograph 2034 applies to all the substances for pharmaceutical use within the given scope.
In addition, the EPC defined clear rules on when a specification for N-nitrosamine impurities should be added to the Tests section of an active substance monograph. Such a specification will therefore only be included when the N-nitrosamine is a process-related impurity, i.e. generated during synthesis of the substance, or a degradation impurity arising from its storage. The test and limit will be included in an individual monograph if the impurity has been detected in several sources of the substance and once a limit approved by competent authorities becomes available.
The EPC also agreed not to address the following types of potential N-nitrosamine contamination in individual monographs as they are assumed to be covered elsewhere:
- impurities resulting from manufacturing practices during synthesis of the API (recovered or recycled materials, cross-contamination);
- impurities potentially generated during the manufacture or storage of the medicinal product;
- potential risk of future transformation into a nitrosamine (e.g. secondary amines).
Regarding medicinal products
Where monographs on medicinal products are concerned, the EPC agreed that it was not appropriate to introduce a specification in the Tests section or a statement in the Production section, except in specific and justified cases, since the risk of N-nitrosamine formation depends to a great extent on the composition of the medicinal product and the conditions under which it is manufactured. The N-nitrosamines section of the general monograph Pharmaceutical preparations (2619) is considered to fully address this risk.
Impact of the new strategy on existing individual monographs on active substances
In view of this new strategy, revised versions of the five individual sartan monographs (Valsartan, Irbesartan, Candesartan cilexetil, Olmesartan medoxomil and Losartan potassium), from which the Production section referring to N-nitrosamines had been deleted, were submitted to and adopted by the EPC at the same session. The EPC considered that the information in the Production section of these individual monographs was redundant since it was already covered by the general requirement for N-nitrosamines in revised general monograph 2034. Although the statement has been removed from the individual monographs, the requirement to control these impurities therefore persists. The revised monographs will be published in European Pharmacopoeia (Ph. Eur.) Supplement 11.6 and implemented as from 1 January 2025.
The two other individual monographs that include a Production section (Clopamide and Dalteparin sodium) were considered to be more complex. The EPC therefore agreed to add them to the work programme of the relevant group of experts to bring them in line with the new strategy. Stakeholders will be informed in due time about the progress on these texts through the usual revision process.
Monographs that already describe a test and a limit for the control of a specific N-nitrosamine impurity (e.g. Gliclazide, Molsidomine, Indapamide and Trolamine) were not modified but will be re-examined should any new information from competent authorities become available. The EPC also decided to monitor any other monographs where a test and specifications for a N-nitrosamine impurity might be introduced in the future, in the event that new data emerges. Stakeholders are encouraged to share any relevant data and information they have with the European Directorate for the Quality of Medicines & HealthCare (EDQM).
See also: