Blood Quality Management programme (B-QM)
Implementation of a quality management system (QMS) in European blood establishments (BEs) is a requirement under EU Directives 2002/98/EC and 2005/62/EC and is also prescribed in the Council of Europe's Guide to the preparation, use and quality assurance of blood components.
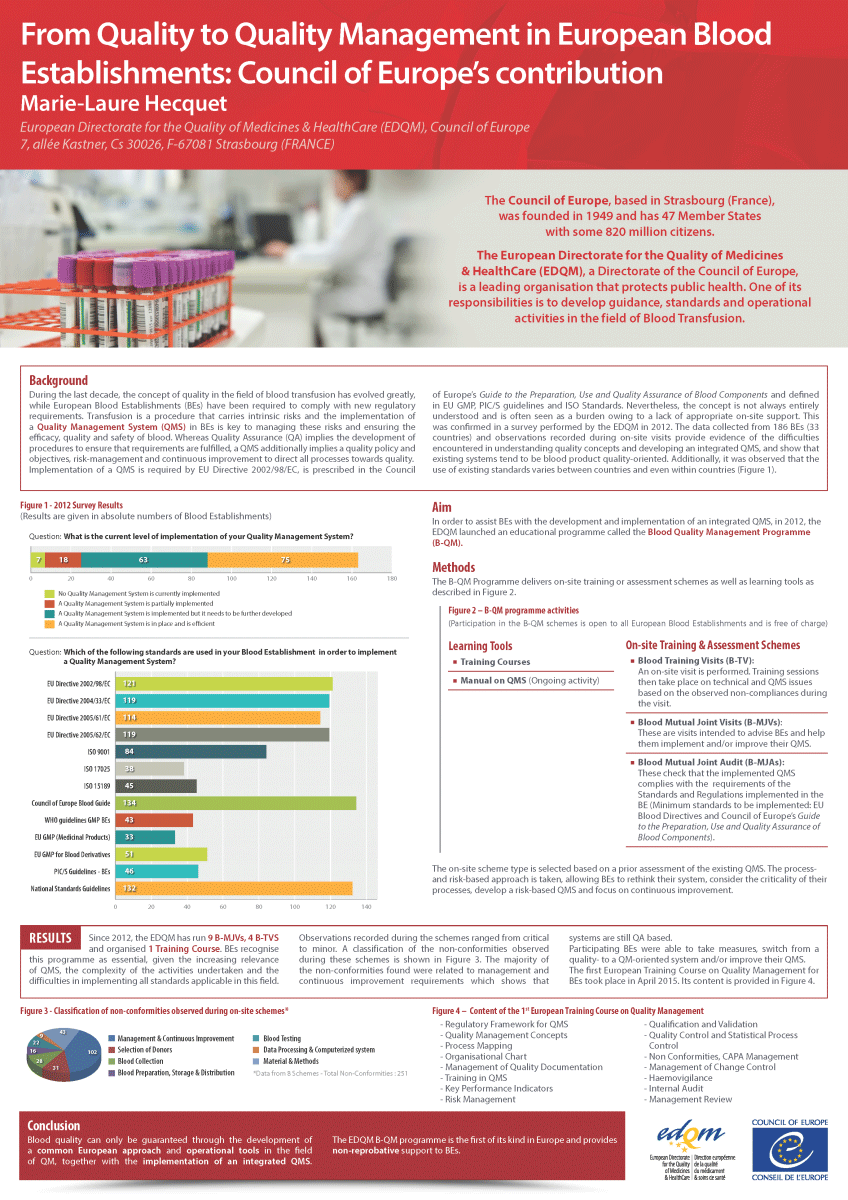
However, the implementation of a QMS is often perceived as a burden, and the concept of a QMS itself is not always fully understood, mainly due to a lack of appropriate support. This was confirmed in a survey performed by the EDQM in 2012, in which data collected from 186 blood establishments located in 33 countries provided evidence about the difficulties encountered by BEs in understanding quality concepts, implementing requirements and developing an integrated QMS. The survey results also highlighted that the systems in place in BEs were mostly blood component quality-oriented. In addition, the reported extent of the use of existing standards and guidelines varied between and even within countries. This was confirmed by observations made during on-site visits to BEs. It was this situation and to help BEs overcome their difficulties, that prompted the development of the Blood Quality Management (B-QM) Programme.
The B-QM Programme started with a pilot phase in April 2012 that ran until December 2013. The outcome of the pilot phase was assessed and the necessary adjustments made before the full Programme was launched.
The B-QM Programme is aimed at supporting European BEs in developing, implementing and improving their QMS. It also takes into account the specificities of the blood transfusion field.
The B-QM Programme is an assistance and educational programme delivering virtual / on-site training and assessment schemes open to BEs from the European Union and Council of Europe member states.
Activities
On-site and virtual schemes
Blood Tailored Expert Support (B-TES)
B-TES aims at providing tailored support to the staff of the requesting BE on technical and / or QMS issues raised prior to the scheme, and recommendations on how to address these issues from a quality management perspective.
B-TES is offered in virtual format.
Blood Training Visit (B-TV)
The B-TV starts with a visit to the BE. Issues encountered by the BE and non-compliances observed during the visit are collected and training is then provided to BE staff on technical and QMS topics.
Blood Mutual Joint Visit (B-MJV)
B-MJVs are intended to advise the BEs and help them implement and / or improve their QMS. The QMS under development in the BE is observed and recommendations are provided.
Blood Mutual Joint Audit (B-MJA)
A B-MJA is intended to check that the QMS implemented in the BE complies with the requirements of the standards and regulations to be followed (minimum standards used: EU Blood legislation and Council of Europe’s Guide to the preparation, use and quality assurance of blood components).
As of 2022, B-MJAs, B-MJVs and B-TVs can be offered in virtual format as an alternative to the on-site schemes.
All schemes are carried out by peers, experts from European BEs, who provide and share their experience and knowledge.
Training courses and conferences on Quality Management
As part of the B-QM Programme, the EDQM organises training courses and conferences with the aim of sharing best practices in the blood transfusion field.
Achievements
Between the launch of the B-QM Programme in April 2012 and December 2022, 36 schemes were carried out, during which European BE staff received training and support for the development of their QMS. It is an encouraging sign that an increasing number of BEs are applying to take part in the schemes.
Since 2015, the following training courses and conferences have been organised:
- European Training Course on Quality Management for European blood establishments, comprising:
- European Training Course on Quality Management, 14-17 April 2015, Strasbourg, France
- European Training Course on Quality Management, 27-30 September 2016, Skopje, North Macedonia
- European Training Course on Quality Management, with a focus on statistical process control (SPC) in blood establishments, 23-24 October 2018, Strasbourg, France
- European Training Course on Quality Management, with a focus on quality risk management (QRM) in blood establishments, November 2021, virtual
- European Training Course on Quality Management with a focus on audit practices in blood establishments, September-October 2022, virtual
- European Conference on “Sharing best practices: quality risk management, change control, validation and qualification in Blood Establishments”, 17-19 October 2017, Strasbourg, France
- “Keeping up with Reality and Quality: A Challenge for European blood establishments”, 27-29 October 2020, virtual

Participation in the B-QM schemes
Participation in B-QM schemes provides European BEs with an objective means of implementing, improving and / or monitoring their Quality Management System.
These schemes are open to all European BEs and are free of charge for the participants.
To apply for a scheme, please complete this form.
If you have any questions or wish to receive any further information, please do not hesitate to contact us.
Past events
- Audit Practices in Blood Establishments, September-October 2022, virtual
- Quality Risk Management, November 2021, virtual
- Keeping up with Reality and Quality: A Challenge for European Blood Establishments, 27-29 October 2020, virtual
- Training Course on Statistical Process Control (SPC) for Blood Establishments, 23-24 October 2018, Strasbourg, France
- European Conference on sharing best practices: quality risk management, change control, validation and qualification in Blood Establishments, 17-19 October 2017, Strasbourg, France
- 2nd European Training Course on Quality Management for European Blood Establishments, 27-30 September 2016, Skopje, North Macedonia
- 1st European Training Course on Quality Management for European Blood Establishments, 14-17 April 2015, Strasbourg, France